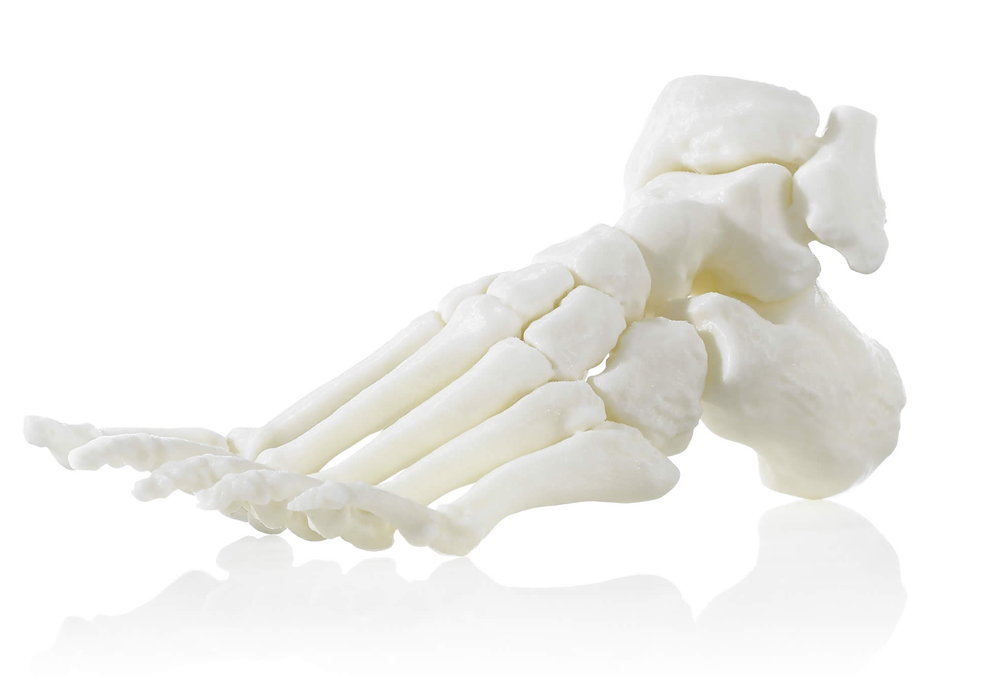
Available with:
Fortus 450mc
/
F900
abs-m30i
– Biocompatible
– High-strength
– Sterilizable
Potential applications:
– Medical uses
–
Food handling
–
Pharmaceutical handling
–
Surgical planning models and tools
Material Description
ABS M30i is an ISO certified FDM 3D printing material. ABS-M30i is high strength and suited for the medical, pharmaceutical and food packaging industries. Parts manufactured with ABS-M30i are biocompatible (ISO 10993)* and can be gamma or EtO sterilized. In addition, ABS M30i has passed ISO 18562 testing for gas and airway parts for use in respiratory and ventilation medical devices. When combined with Fortus® 3D Printers, ABS-M30i gives you biocompatible parts with excellent mechanical properties for conceptual modeling, functional prototyping, manufacturing tools, and production parts applications.
Behavior and Use:
IMPACT toughness
139 J/m (ZX Axis)
HDT @ 66 psi
96° C
tensile modulus
2,400 MPa
TENSILE STRENGTH, yield
36 MPa
Additional 3D Printing Resources
AWARD-WINNING TECHNICAL SUPPORT
GoEngineer’s extensive technical knowledge can assist with your additive manufacturing needs. Our Award winning team is ready to help. Reach out and see why GoEngineer is the #1 reseller of SOLIDWORKS and Stratasys systems in the world!
3D Printing Courses
Learn to utilize all features and tools of Stratasys commercial 3D printers with GoEngineer additive manufacturing on-boarding training and 3D printing courses. Take advantage of our 3D printing team of experts to help launch all your 3D printing capabilities.
3D PrintING SERVICES
No matter the size, quantity, or complexity of part(s) needed, GoEngineer can help you! Take advantage of our 3D Printing Services to help your organization produce the best parts and prototypes available on the market.
Additional Resources
Take Advantage of GoEngineer’s Extensive Knowledge Base and Resources

Find a Solution
Our robust Knowledge Base contains over 12,000 resources to help answer your product design questions. From basic CAD questions to in-depth guides and tutorials, find your solution here. Find a Solution

PROFESSIONAL TRAINING
Improve your skills with professional training and certifications in SOLIDWORKS, CAM, 3D Printing, and 3D Scanning offered four ways: self-paced, online, on-site, or in-classroom. Certified Training Courses

BLOG
#1 Technical Resource Worldwide - Right at your fingertips. Search or browse through hundreds of SOLIDWORKS tips & tricks, additive manufacturing product developments, announcements, how-to guides, and tutorials. Blog

YouTube Channel
Our YouTube channel hosts hundreds of educational tutorials, product demonstrations, recorded webinars, and best practices for all of our products and services. GoEngineer's YouTube Channel

ONLINE STORE
Order 3D printing materials and consumables, enroll in SOLIDWORKS training classes, and buy official GoEngineer gear directly from our online store. Online Store

WEBINARS
Our engineering webinars are hosted by some of the top experts in the industry. They are always recorded, always free, and always offer a live Q&A. WEBINARS
3D Printing Services
Need to 3D print a part? Our Additive Manufacturing experts will 3D print your part and deliver it to you using the latest technology on one of our professional FDM, PolyJet and SL 3D printers. 3D Printing Services